ESG – Environmental, Social, and Governance
- Based on our corporate values, we have an organizational culture which appreciates and actively promotes diversity, equity, and inclusion.
- Our research and development process is designed in a way to provide our employees with a safe work environment, to protect the safety of our clinical participants, and to reduce environmental impact.
- We strive to fulfil our social responsibilities through fair and consistent practices and transparent communication with our employees and external stakeholders.
Initiatives

NIT became a participant of the United Nations Global Compact initiative since September 2022. NIT accepts and will take actions to support the Ten Principles of the UN Global Compact and the Sustainable Development Goals (SDGs).
Core Company Values
-
Respect
We strive to always be humble and show respect towards others. This means that we celebrate their successes and empathize with their failures. We appreciate each other’s similarities as well as differences and meet others where they are. Respect is the ruling value.
-
Self-motivation
At the core of our values is hard work and dedication. Through an inner desire to be better, we strive to cultivate and nurture self-motivation in ourselves and in others around us every single day. Positivity begets positivity.
-
Responsibility
Being responsible for our thoughts and actions is at the heart of who we are. We take responsibility seriously. We hold ourselves and our business partners to a very high standard.
Environment l Biosafety
We strive to reduce environmental impact.
-

- By structuring a responsible system for our employees to work from home, we reduce the portion of our carbon footprint caused by commuting.
-

- Complying biosafety management for waste boxes and waste liquid during manufacturing and testing in laboratories.
- Disposing waste & waste liquid with a bio-specialized collector.
-

- Establishing biosafety management guidelines and lab safety policies.
- Providing organizational support to ensure best and safe R&D practices.
- Providing training to R&D staff to ensure sustainable biosafety.
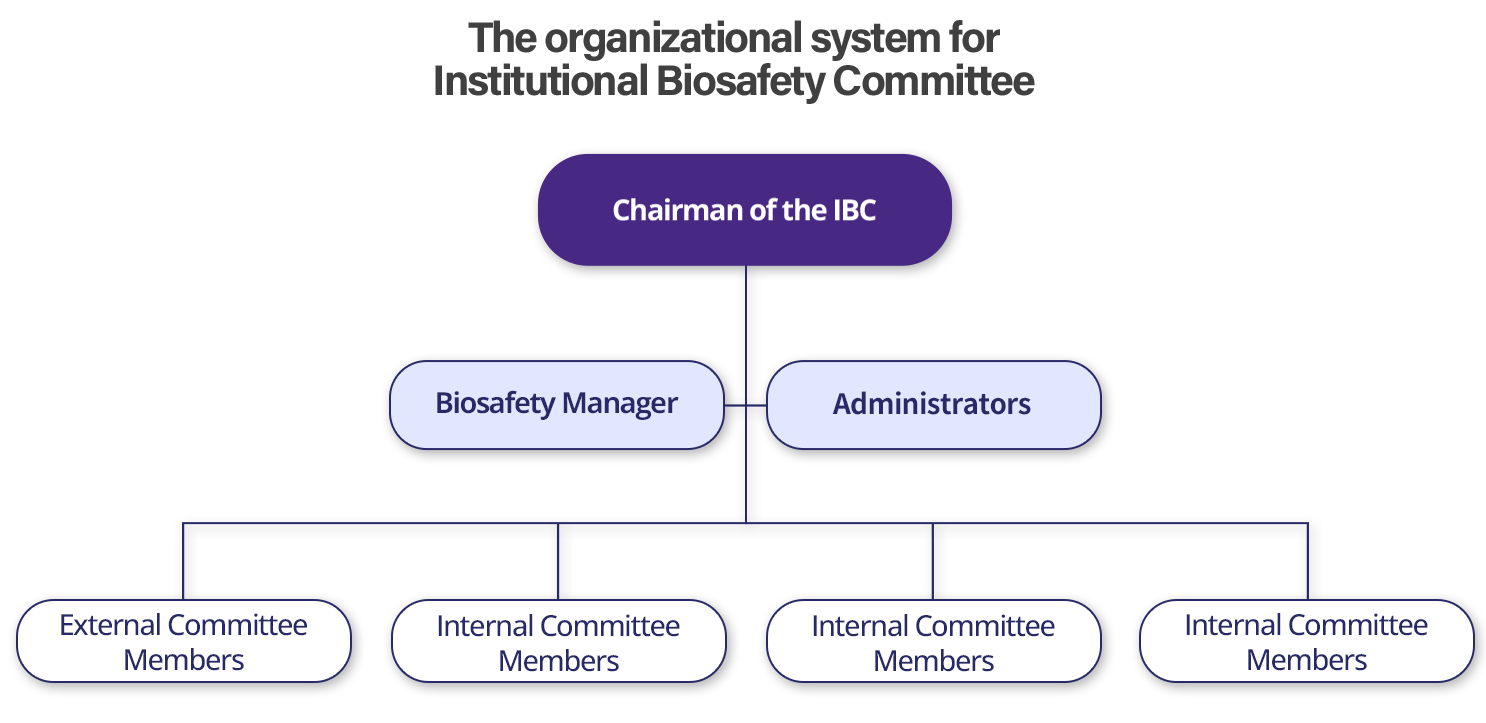
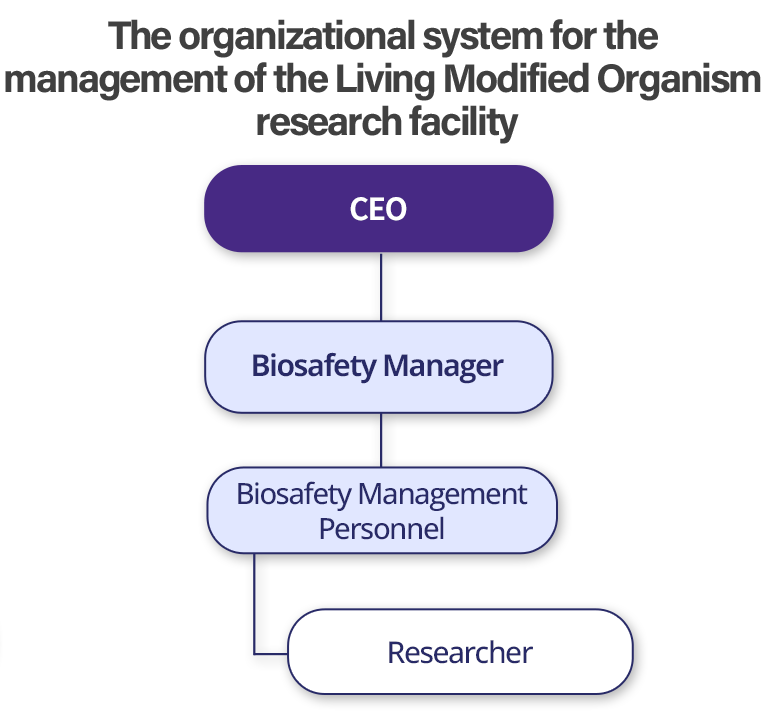
Biosafety Management Structure
Our Employees l
Diversity, Equity, and Inclusion
NeoImmuneTech values diversity, equity, and inclusion and prohibits discrimination on the basis of race, color, religion, age, sex, national origin, disability status, genetics, protected veteran status, sexual orientation, gender identity or expression, or any other characteristic protected by federal, state, or local laws.
We ensure equal employment opportunities(EEO) by establishing policies and procedures that are compliant and follow best practices and interpret policies in a fair and consistent manner. Here are some examples of our proactive approach to promote EEO:
- We select, interview, and hire the most qualified individuals for the positions through fair, consistent, and best talent acquisition practices.
- We prevent harassment and discrimination in the workplace by educating our employees and by having in place a Code of Conduct and an Employee Resolution Program.
- We promote and reward our employees based on objective performance evaluations.
Social Contributions l
Community Relations
NeoImmuneTech is operating the ‘Pohang Innovation Salon (PIS)’ to share information and experiences about bio and healthcare industry with POSTECH in Pohang, where the research institute is located.
- After the first PIS in May 2021, lectures were held by about 30 experts.
- About 400 industry experts and researchers participated to exchange knowledge about the bio industry and business.
Safety l Laboratory
& Data Security
NeoImmuneTech has established policies and procedures to ensure workplace safety.
- Workplace Safety and Security
- Drug-Free Workplace
- Alcohol-Free Workplace
- Smoke-Free Workplace
We have established information security policies and practices to ensure the security of all sensitive, proprietary, and confidential information.
- General Data Protection Regulation (GDPR) compliance implemented for clinical data safety.
- Mandatory security pledges and mandatory information security programs.
Product Quality l
Global Compliance
NeoImmuneTech, as a biotech company dedicated to development of novel immunotherapeutics, we comply with all applicable laws and regulations for the health and safety of patients.
- Compliance with GCP (Good Clinical Practice) to enhance reliability and ensure patients safety.
- Manufacture according to cGMP/GMP (current Good Manufacturing Practice) management procedures through a contract manufacturing organization (CMO) approved in advance by regulatory authorities such as the FDA and EMA.
NeoImmuneTech provides this link as a courtesy and does not control the content of the site you are about to enter.
Do you wish to continue?